颜标 博士/研究员(复旦大学附属眼耳鼻喉科医院)
报告主题:circRNA在眼科血管疾病中的调控作用及其机制

嘉宾简介:
中国医师协会眼科医师分会委员、上海市眼科学分会委员和上海市生物工程学会委员。主要从事眼科血管性疾病的发病机制和干预策略研究,发现了视网膜血管内皮病变的非编码RNA介导机制,揭示了视网膜血管病变和神经病变协同调控的非编码RNA介导机制。目前已主持国家自然基金3项,省部级项目4项;以第一作者或通讯作者(含共同)发表SCI论文50余篇,涵盖8篇中科院1区的杂志和3篇眼科领域的权威杂志(IOVS);代表论文发表在Circulation, Circ Res, EMBO Mol Med和Genet Med等期刊;曾获得上海市青年拔尖人才和上海市晨光学者的称号;应邀为Nucleic Acids Res, Theranostics, Mol Ther, Cell Death Dis, Exp Eye Res和 IOVS等期刊审稿。
代表性成果介绍:
PNAS:cPWWP2A改善糖尿病引起的视网膜病变
2019年4月9日,PNAS杂志发表了一项circRNA的重要研究工作,报道发现cPWWP2A可改善糖尿病引起的视网膜病变,文章的通讯作者是复旦大学上海医学院附属耳鼻喉眼科医院的颜标教授,赵晨教授和南京医科大学附属眼科医院的蒋沁教授。
该研究显示糖尿病应激上调视网膜周细胞,而不是内皮细胞中cPWWP2A的表达水平。体外研究表明,cPWWP2A充当内源性miR-579的海绵,调控Angiopoietin 1/Occludin/SIRT1基因的表达,直接调节周细胞生物学功能,而通过携带cPWWP2A的外泌体间接调节ECs生物学功能,促进ECs的迁移和血管形成能力。体内研究表明,cPWWP2A过表达或抑制miR-579表达可减轻糖尿病引起的视网膜血管功能障碍。 相反,抑制cPWWP2A表达或过表达miR-579会加重视网膜血管功能障碍。这项研究揭示了周细胞和ECs “对话”的新机制,干预cPWWP2A或miR-579的表达可为治疗糖尿病微血管并发症提供治疗策略。(参考文献[1])
推荐阅读:cPWWP2A改善糖尿病引起的视网膜病变
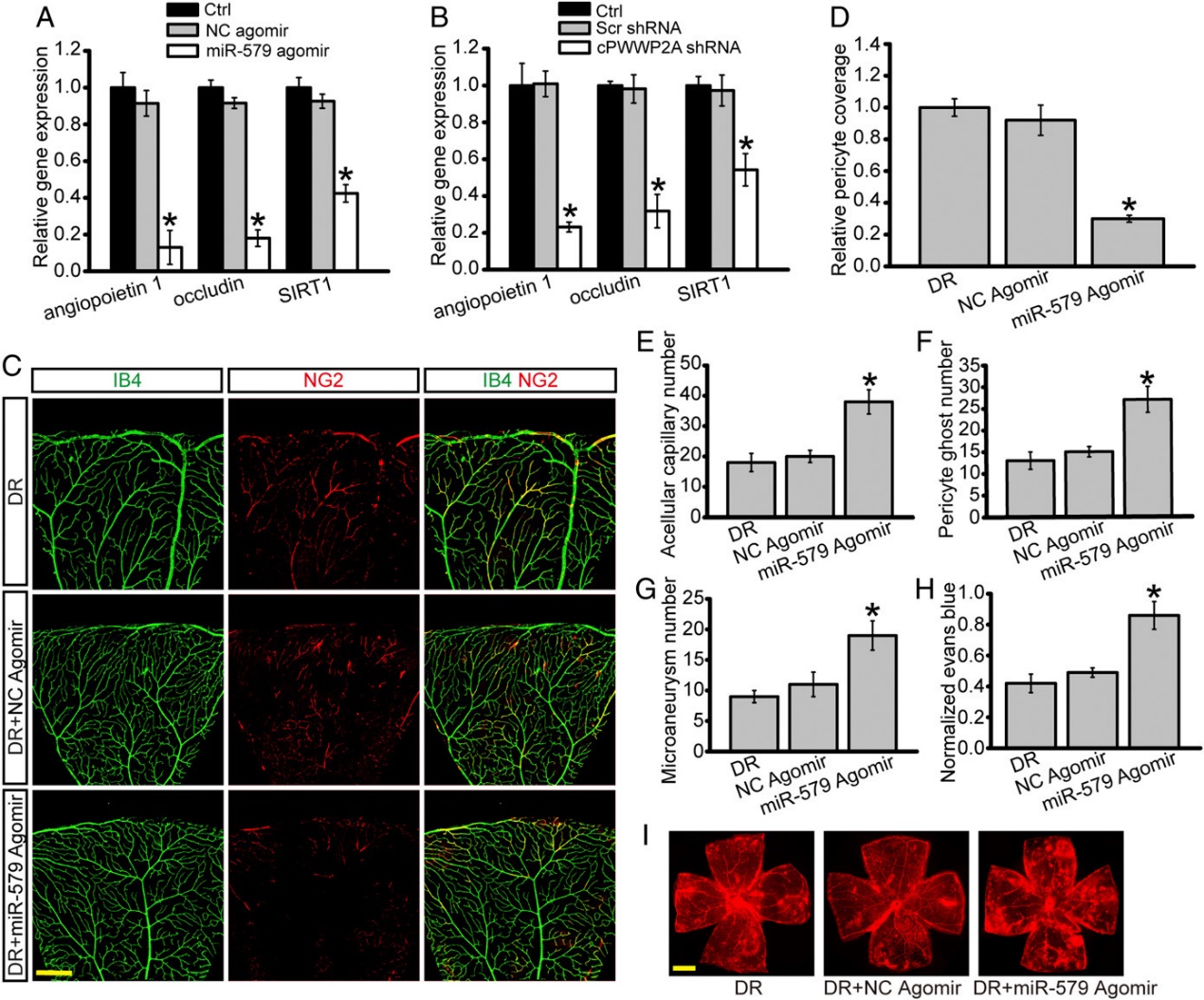
体内cPWWP2A对于视网膜血管功能障碍的影响(参考文献[1])
Theranostics: cZNF609通过竞争性吸附miR-615调控视网膜神经退化
2018年5月24日,Theranostics杂志发表了一项circRNA的研究工作,报道cZNF609通过竞争性吸附miR-615调控视网膜神经退化。文章的通讯作者是复旦大学上海医学院附属耳鼻喉眼科医院的颜标教授和孙兴怀教授。
以渐进性视网膜神经变性导致的视力损伤是青光眼形成的主要原因。环状RNA是一类内源性RNA,可调节真核生物中的基因表达。该研究中,研究者分析了cZNF609在视网膜神经变性诱导的青光眼中的作用。方法:qRT-PCR和Sanger测序法检测cZNF609在视网膜神经变性过程中的表达模式。免疫荧光染色检测cZNF609沉默对体内视网膜神经变性的影响。 MTT法,Ki67染色法和PI染色法检测cZNF609沉默对视网膜胶质细胞的影响及体外RGC功能。生物信息学分析,RNApulldown实验测定揭示cZNF609介导的视网膜神经变性的机制。结果:cZNF609表达在视网膜神经变性过程中显着上调。 cZNF609沉默减少了视网膜反应性神经胶质细胞增生和神经胶质细胞活化,并促进了RGC(视网膜神经节细胞)在体内的存活。 cZNF609沉默直接调节Müller细胞功能,但间接调节RGC功能。 cZNF609内源性吸附miR-615,起到隔离和抑制miR-615活性的作用,导致METRN增加。 METRN过表达可部分挽救cZNF609沉默介导的对视网膜神经胶质细胞增殖的抑制作用。结论:干预cZNF609表达是视网膜神经变性的一种有前途的治疗策略。(参考文献[9])

Circulation:circHIPK3分子在糖尿病视网膜血管障碍中的作用
2017年8月31日,著名循环学杂志Circulation在线发表了复旦大学眼耳鼻喉科医院颜标教授与赵晨教授为共同通讯作者的文章,介绍发现circular HIPK3在糖尿病视网膜血管障碍中的作用。
本文发现circHIPK3可以竞争性结合miR-30a-3p、miR-30d-3p和miR-30e-3p,进而影响下游基因VEGFC/WNT2/FZD4,在糖尿病视网膜血管障碍中的作用。(参考文献[15])
推荐阅读:连环炮!Circulation杂志再发重要circRNA文章

Theranostics: 干扰 circZNF609改善血管内皮功能障碍
2017年7月8日,Theranostics发表了复旦大学上海眼耳鼻喉医院的颜标教授和南京医科大学附属眼科医院的蒋沁教授为共同通讯作者的文章,报道发现干扰 circZNF609改善血管内皮功能障碍。文章作者使用视网膜脉管系统来确定circRNA在血管功能障碍中的作用。发现cZNF609在体内和体外高葡萄糖和低氧应激下显着上调。 干扰cZNF609减少了视网膜血管丢失并抑制了体内病理性血管生成。干扰cZNF609增加内皮细胞迁移和管形成,并在体外保护内皮细胞免受氧化应激和缺氧应激。

EMBO Molecular Medicine:非编码RNA MALAT1通过CREB信号传导视网膜神经变性
2016年4月1日,EMBO Molecular Medicine杂志发表了复旦大学上海眼耳鼻喉医院的颜标教授和南京医科大学附属眼科医院的蒋沁教授为共同通讯作者的文章,报道非编码RNA MALAT1通过CREB信号传导视网膜神经变性。
本文中作者探索了MALAT1在视网膜变性中的作用,结果显示在应激刺激时视网膜Müller细胞和原发性视网膜神经节细胞(RGC)中MALAT1表达增高。敲低MALAT1会导致反应性胶质细胞减少,降低Müller细胞活化。MALAT1可以结合CREB,抑制后者与PP2A的结合,维持CREB的磷酸化状态。临床和动物实验表明,MALAT1功能障碍与神经退行性病变和几种人类疾病有关。(参考文献[24])
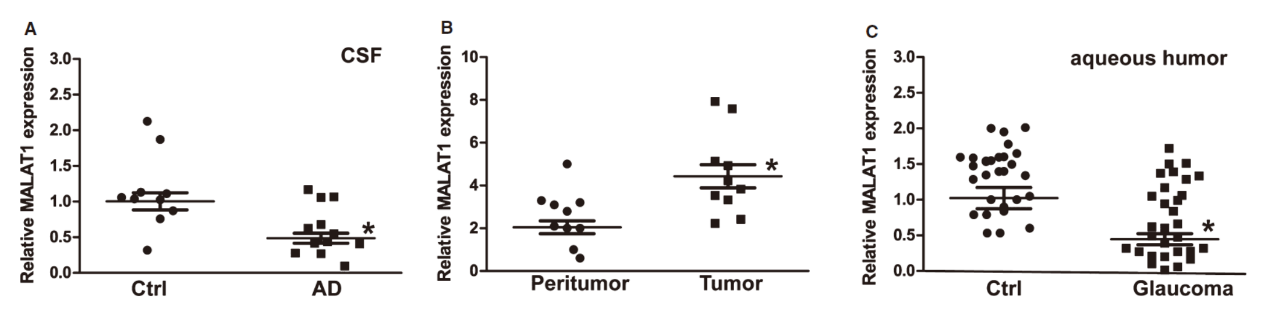
神经系统疾病中MALAT1表达情况(参考文献[24])
Circulation Research:非编码RNA MIAT参与病理性血管形成
2015年3月27日,Circulation Research杂志在线发表了南京医科大学眼科医院颜标和蒋沁为共同通讯作者的文章,报道非编码MIAT在病理性血管形成中的作用。QPCR检测表明MIAT在糖尿病视网膜病变中增高,高糖培养内皮细胞中也呈现增高状态。干扰MIAT可抑制内皮细胞增殖,迁移和管形成。机制方面,MIAT可作为内源竞争性RNA结合miR-150-5p发挥作用。(参考文献[29])

代表性研究成果列表
1. Liu C, Ge HM, Liu BH, Dong R, Shan K, Chen X, Yao MD, Li XM, Yao J, Zhou RM, Zhang SJ, Jiang Q*, Zhao C*, Yan B*. Targeting pericyte-endothelial cell crosstalk by circular RNA-cPWWP2A inhibition aggravates diabetes-induced microvascular dysfunction. Proc Natl Acad Sci U S A. 2019 Apr 9;116(15):7455-7464. doi: 10.1073/pnas.1814874116
2. Sun JG, Jiang Q, Zhang XP, Shan K, Liu BH, Zhao C, Yan B*. Mesoporous silica nanoparticles as a delivery system for improving antiangiogenic therapy. Int J Nanomedicine. 2019 Feb 25;14:1489-1501. doi: 10.2147/IJN.S195504
3. Huang J, Gu S, Chen M, Zhang SJ, Jiang Z, Chen X, Jiang C, Liu G, Radu RA, Sun X, Vollrath D, Du J, Yan B*, Zhao C*. Abnormal mTORC1 signaling leads to retinal pigment epithelium degeneration. Theranostics. 2019 Jan 30;9(4):1170-1180. doi: 10.7150/thno.26281
4. Yao J, Hu LL, Li XM, Shan K, Zhou RM, Ge HM, Yao MD, Jiang Q*, Zhao C*, Yan B*. Comprehensive circular RNA profiling of proliferative vitreoretinopathy and its clinical significance. Toxicol Appl Pharmacol. 2019 Apr 1;368:1-17. doi: 10.1016/j.taap.2019.02.008
5. Liu X, Liu C, Shan K, Zhang S, Lu Y, Yan B*, Luo Y*. Long Non-Coding RNA H19 Regulates Human Lens Epithelial Cells Function. Cell Physiol Biochem. 2018;50(1):246-260. doi: 10.1159/000494003.
6. Zhang XP, Sun JG, Yao J, Shan K, Liu BH, Yao MD, Ge HM, Jiang Q*, Zhao C*, Yan B*. Effect of nanoencapsulation using poly (lactide-co-glycolide) (PLGA) on anti-angiogenic activity of bevacizumab for ocular angiogenesis therapy. Biomed Pharmacother. 2018 Nov;107:1056-1063. doi: 10.1016/j.biopha.2018.08.092
7. Chen X, Wang X, Jiang C, Xu M, Liu Y, Qi R, Qi X, Sun X, Xie P, Liu Q, Yan B*, Sheng X*, Zhao C*. IFT52 as a Novel Candidate for Ciliopathies Involving Retinal Degeneration. Invest Ophthalmol Vis Sci. 2018 Sep 4;59(11):4581-4589. doi: 10.1167/iovs.17-23351.
8. Li XM, Ge HM, Yao J, Zhou YF, Yao MD, Liu C, Hu HT, Zhu YX, Shan K, Yan B*, Jiang Q*. Genome-Wide Identification of Circular RNAs as a Novel Class of Putative Biomarkers for an Ocular Surface Disease. Cell Physiol Biochem. 2018;47(4):1630-1642. doi: 10.1159/000490982.
9. Wang JJ, Liu C, Shan K, Liu BH, Li XM, Zhang SJ, Zhou RM, Dong R, Yan B*, Sun XH*. Circular RNA-ZNF609 regulates retinal neurodegeneration by acting as miR-615 sponge. Theranostics. 2018 May 24;8(12):3408-3415. doi: 10.7150/thno.25156
10. Chen X, Sheng X, Liu Y, Li Z, Sun X, Jiang C, Qi R, Yuan S, Wang X, Zhou G, Zhen Y, Xie P, Liu Q, Yan B*, Zhao C*. Distinct mutations with different inheritance mode caused similar retinal dystrophies in one family: a demonstration of the importance of genetic annotations in complicated pedigrees. J Transl Med. 2018 May 29;16(1):145. doi: 10.1186/s12967-018-1522-7.
11. Wang JJ, Shan K, Liu BH, Liu C, Zhou RM, Li XM, Dong R, Zhang SJ, Zhang SH, Wu JH, Yan B*. Targeting circular RNA-ZRANB1 for therapeutic intervention in retinal neurodegeneration. Cell Death Dis. 2018 May 1;9(5):540. doi: 10.1038/s41419-018-0597-7
12. Zhu YX, Yao J, Liu C, Hu HT, Li XM, Ge HM, Zhou YF, Shan K, Jiang Q*, Yan B*. Long non-coding RNA MEG3 silencing protects against light-induced retinal degeneration. Biochem Biophys Res Commun. 2018 Feb 19;496(4):1236-1242. doi: 10.1016/j.bbrc.2018.01.177.
13. Zhang SJ, Chen X, Li CP, Li XM, Liu C, Liu BH, Shan K, Jiang Q*, Zhao C*, Yan B*. Identification and Characterization of Circular RNAs as a New Class of Putative Biomarkers in Diabetes Retinopathy. Invest Ophthalmol Vis Sci. 2017 Dec 1;58(14):6500-6509. doi: 10.1167/iovs.17-22698.
14. Chen X, Jiang C, Qin B, Liu G, Ji J, Sun X, Xu M, Ding S, Zhu M, Huang G, Yan B, Zhao C. LncRNA ZNF503-AS1 promotes RPE differentiation by downregulating ZNF503 expression. Cell Death Dis. 2017 Sep 7;8(9):e3046. doi: 10.1038/cddis.2017.382.
15. Shan K, Liu C, Liu BH, Chen X, Dong R, Liu X, Zhang YY, Liu B, Zhang SJ, Wang JJ, Zhang SH, Wu JH, Zhao C*, Yan B*. Circular Noncoding RNA HIPK3 Mediates Retinal Vascular Dysfunction in Diabetes Mellitus. Circulation. 2017 Oct 24;136(17):1629-1642. doi: 10.1161/CIRCULATIONAHA.117.029004
16. Liu C, Yao MD, Li CP, Shan K, Yang H, Wang JJ, Liu B, Li XM, Yao J, Jiang Q*, Yan B*. Silencing Of Circular RNA-ZNF609 Ameliorates Vascular Endothelial Dysfunction. Theranostics. 2017 Jul 8;7(11):2863-2877. doi: 10.7150/thno.19353. eCollection 2017.
17. Chen X, Sheng X, Zhuang W, Sun X, Liu G, Shi X, Huang G, Mei Y, Li Y, Pan X, Liu Y, Li Z, Zhao Q, Yan B*, Zhao C*. GUCA1A mutation causes maculopathy in a five-generation family with a wide spectrum of severity. Genet Med. 2017 Aug;19(8):945-954. doi: 10.1038/gim.2016.217
18. Shan K, Li CP, Liu C, Liu X, Yan B*. RNCR3: A regulator of diabetes mellitus-related retinal microvascular dysfunction. Biochem Biophys Res Commun. 2017 Jan 22;482(4):777-783. doi: 10.1016/j.bbrc.2016.11.110
19. Liu C, Li CP, Wang JJ, Shan K, Liu X, Yan B*. RNCR3 knockdown inhibits diabetes mellitus-induced retinal reactive gliosis. Biochem Biophys Res Commun. 2016 Oct 14;479(2):198-203. doi: 10.1016/j.bbrc.2016.09.032.
20. Wang YN, Shan K, Yao MD, Yao J, Wang JJ, Li X, Liu B, Zhang YY, Ji Y, Jiang Q*, Yan B*. Long Noncoding RNA-GAS5: A Novel Regulator of Hypertension-Induced Vascular Remodeling. Hypertension. 2016 Sep;68(3):736-48. doi: 10.1161/HYPERTENSIONAHA.116.07259.
21. Jiang Q, Shan K, Qun-Wang X, Zhou RM, Yang H, Liu C, Li YJ, Yao J, Li XM, Shen Y, Cheng H, Yuan J, Zhang YY, Yan B*. Long non-coding RNA-MIAT promotes neurovascular remodeling in the eye and brain. Oncotarget. 2016 Aug 2;7(31):49688-49698. doi: 10.18632/oncotarget.10434.
22. Yang H, Liu C, Zhou RM, Yao J, Li XM, Shen Y, Cheng H, Yuan J, Yan B*, Jiang Q*. Piezo2 protein: A novel regulator of tumor angiogenesis and hyperpermeability. Oncotarget. 2016 Jul 12;7(28):44630-44643. doi: 10.18632/oncotarget.10134.
23. Zhou RM, Shen Y, Yao J, Yang H, Shan K, Li XM, Jiang Q*, Yan B*. Nmnat 1: a Security Guard of Retinal Ganglion Cells (RGCs) in Response to High Glucose Stress. Cell Physiol Biochem. 2016;38(6):2207-18. doi: 10.1159/000445576.
24. Yao J, Wang XQ, Li YJ, Shan K, Yang H, Wang YN, Yao MD, Liu C, Li XM, Shen Y, Liu JY, Cheng H, Yuan J, Zhang YY, Jiang Q*, Yan B*. Long non-coding RNA MALAT1 regulates retinal neurodegeneration through CREB signaling. EMBO Mol Med. 2016 Apr 1;8(4):346-62. doi: 10.15252/emmm.201505725
25. Shen Y, Dong LF, Zhou RM, Yao J, Song YC, Yang H, Jiang Q*, Yan B*. Role of long non-coding RNA MIAT in proliferation, apoptosis and migration of lens epithelial cells: a clinical and in vitro study. J Cell Mol Med. 2016 Mar;20(3):537-48. doi: 10.1111/jcmm.12755
26. Dong LF, Yao J, Wang XQ, Shan K, Yang H, Yan B*, Jiang Q*. Lenalidomide, an anti-tumor drug, regulates retinal endothelial cell function: Implication for treating ocular neovascular disorder. Biochem Biophys Res Commun. 2015 Oct 2;465(4):678-84. doi: 10.1016/j.bbrc.2015.08.014
27. Zhou RM, Wang XQ, Yao J, Shen Y, Chen SN, Yang H, Jiang Q*, Yan B*. Identification and characterization of proliferative retinopathy-related long noncoding RNAs. Biochem Biophys Res Commun. 2015 Sep 25;465(3):324-30. doi: 10.1016/j.bbrc.2015.07.120
28. Li YJ, Jiang Q, Cao GF, Yao J*, Yan B*. Repertoires of autophagy in the pathogenesis of ocular diseases. Cell Physiol Biochem. 2015;35(5):1663-76. doi: 10.1159/000373980
29. Yan B*, Yao J, Liu JY, Li XM, Wang XQ, Li YJ, Tao ZF, Song YC, Chen Q, Jiang Q*. lncRNA-MIAT Regulates Microvascular Dysfunction by Functioning as a Competing Endogenous RNA. Circ Res. 2015 Mar 27;116(7):1143-56. doi: 10.1161/CIRCRESAHA.116.305510
30. Yan B*, Wang ZH, Liu JY, Tao ZF, Li XM, Qin J*. Long noncoding RNAs: versatile players in biologcial processes and human disorders. Epigenomics. 2014;6(4):375-9. doi: 10.2217/epi.14.29
31. Xu XD, Li KR, Li XM, Yao J, Qin J*, Yan B*. Long non-coding RNAs: new players in ocular neovascularization. Mol Biol Rep. 2014 Jul;41(7):4493-505. doi: 10.1007/s11033-014-3320-5
32. Yao J, Tao ZF, Li CP, Li XM, Cao GF, Jiang Q*, Yan B*. Regulation of autophagy by high glucose in human retinal pigment epithelium. Cell Physiol Biochem. 2014;33(1):107-16. doi: 10.1159/000356654
33. Yan B*, Tao ZF, Li XM, Zhang H, Yao J, Jiang Q*. Aberrant expression of long noncoding RNAs in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2014 Feb 18;55(2):941-51. doi: 10.1167/iovs.13-13221.
34. Li XM, Wendu RL, Yao J, Ren Y, Zhao YX, Cao GF, Qin J*, Yan B*. Abnormal glutamate metabolism in the retina of aquaporin 4 (AQP4) knockout mice upon light damage. Neurol Sci. 2014 Jun;35(6):847-53. doi: 10.1007/s10072-013-1610-7
35. Yan B*, Yao J*, Tao ZF, Jiang Q. Epigenetics and ocular diseases: from basic biology to clinical study. J Cell Physiol. 2014 Jul;229(7):825-33. doi: 10.1002/jcp.24522.
36. Yan B*, Yao J*, Tao ZF, Jiang Q. Epigenetics and ocular diseases: from basic biology to clinical study. J Cell Physiol. 2014 Jul;229(7):825-33. doi: 10.1002/jcp.24522.
37. Li CP, Yao J, Tao ZF, Li XM, Jiang Q*, Yan B*. Epigallocatechin-gallate (EGCG) regulates autophagy in human retinal pigment epithelial cells: a potential role for reducing UVB light-induced retinal damage. Biochem Biophys Res Commun. 2013 Sep 6;438(4):739-45. doi: 10.1016/j.bbrc.2013.07.097
.png)